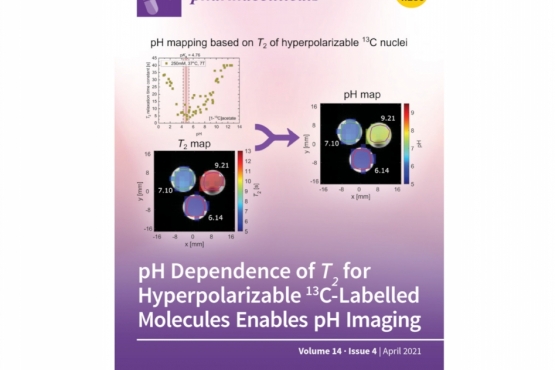
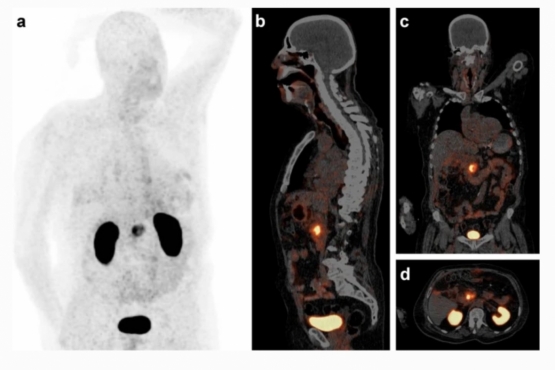
Welcome to the Collaborative Research Center 824 Imaging for Selection, Monitoring and Individualization of Cancer Therapies
The SFB824 represents an interdisciplinary consortium which aims at the development of novel imaging technologies for the selection and monitoring of cancer therapy as important support for personalized medicine.
Post date:
16. June 2021 - 7:22